Products
ePlex RP2 Control M451
The ePlex RP2 Control M451 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of pathogens on the ePlex® Respiratory Pathogen Panel 2 (RP2) performed on the ePlex System (GenMark Diagnostics, Inc.).
ePlex BCID-GN Negative
The ePlex BCID-GN Negative is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Gram-Negative (BCID-GN) Panel performed on the ePlex System (GenMark Diagnostics, Inc.).
ePlex BCID-GN Control M326
The ePlex BCID-GN Control M326 is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Gram-Negative (BCID-GN) Panel performed on the ePlex System (GenMark Diagnostics, Inc.).
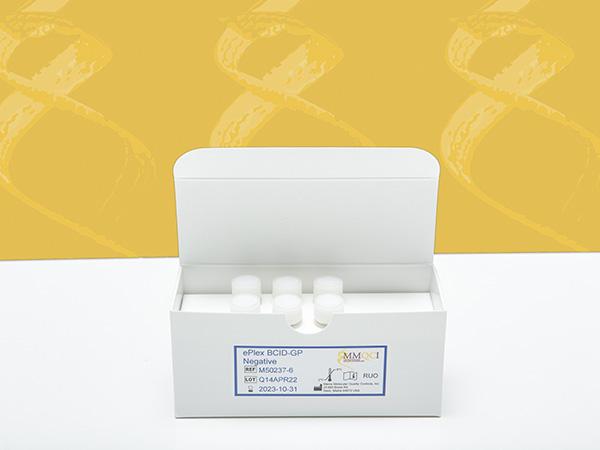
ePlex BCID-GP Negative
The ePlex BCID-GP Negative is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Gram Positive Panel performed on the ePlex System (GenMark Diagnostics, Inc).
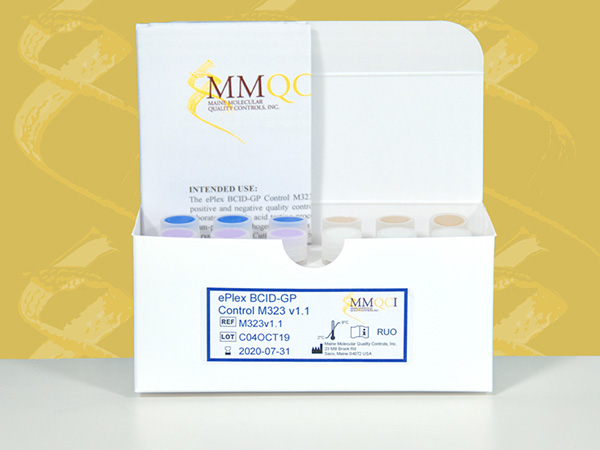
ePlex BCID-GP Control M323 v1.1
The ePlex BCID-GP Control M323 v1.1 is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Gram Positive Panel performed on the ePlex System (GenMark Diagnostics, Inc).
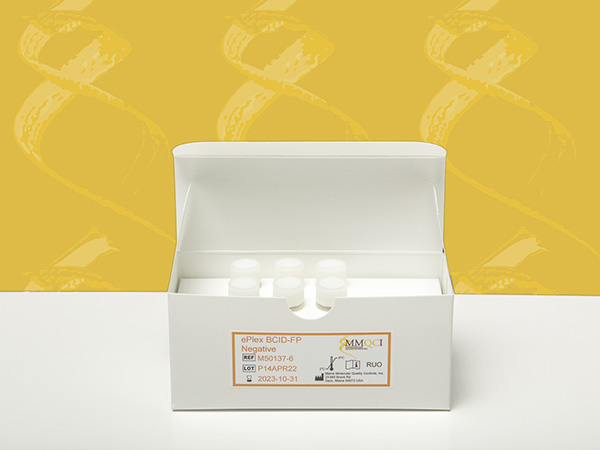
ePlex BCID-FP Negative
The ePlex BCID-FP Negative is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Fungal Pathogen (BCID-FP) Panel performed on the ePlex System (GenMark Diagnostics, Inc).
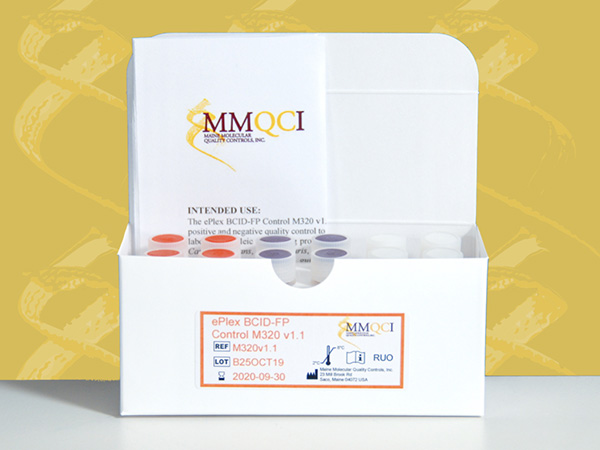
ePlex BCID-FP Control M320 v1.1
The ePlex BCID-FP Control M320 v1.1 is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps of the ePlex® Blood Culture Identification Fungal Pathogen (BCID-FP) Panel performed on the ePlex System (GenMark Diagnostics, Inc).
MMQCI Controls
Maine Molecular Quality Controls, Inc. designs molecular controls for use in inherited disease testing, infectious disease detection, and pharmacogenetics. As experts in quality assurance of laboratory medicine departments, MMQCI produce high calibre controls uniquely suited to monitor all phases of molecular testing as required by best practice and regulations. MMQCI controls provide confidence in test results
Download Our Brochure Now
Please enter your details for more information